Monitoring
Table 5. Recommended monitoring during and after treatment of CanL
| Parameters | Frequency | |
|---|---|---|
| Clinical history and physical examination CBC, biochemical profile ± serum electrophoresis Complete urinalysis ±UPC | Sick treated dogs | Clinically healthy infected dogs |
| After the first month of treatment and then every 3-4 months during the first year. Thereafter, every 6-12 months in dogs fully recovered clinically with treatment. | Every 3-6 months(see figure 2) | |
| Quantitative serology* | Not before 3 months after initial treatment and every 6-12 months. | |
| Real-time PCR (optional)** | At the same time as serology. | |
* Some dogs have a decrease in antibody levels (i.e. on IFAT results at least a 2-3 fold dilutions difference between monitoring samples) associated with clinical improvement within 6-12 months of therapy. An increase in antibody levels (i.e. at least 2-3 fold dilutions difference between monitoring samples) should be interpreted as a marker of disease relapse, especially following the discontinuation of treatment.
** Sensitivity of PCR assays relies considerably on the type and number of tissues evaluated. Bone marrow, lymph node, spleen, and cutaneous lesions are better targets for Leishmania detection than whole blood. Testing two to three samples of different tissues are recommended for a more accurate diagnosis.
Figure 2. Recommended monitoring during and after treatment of CanL
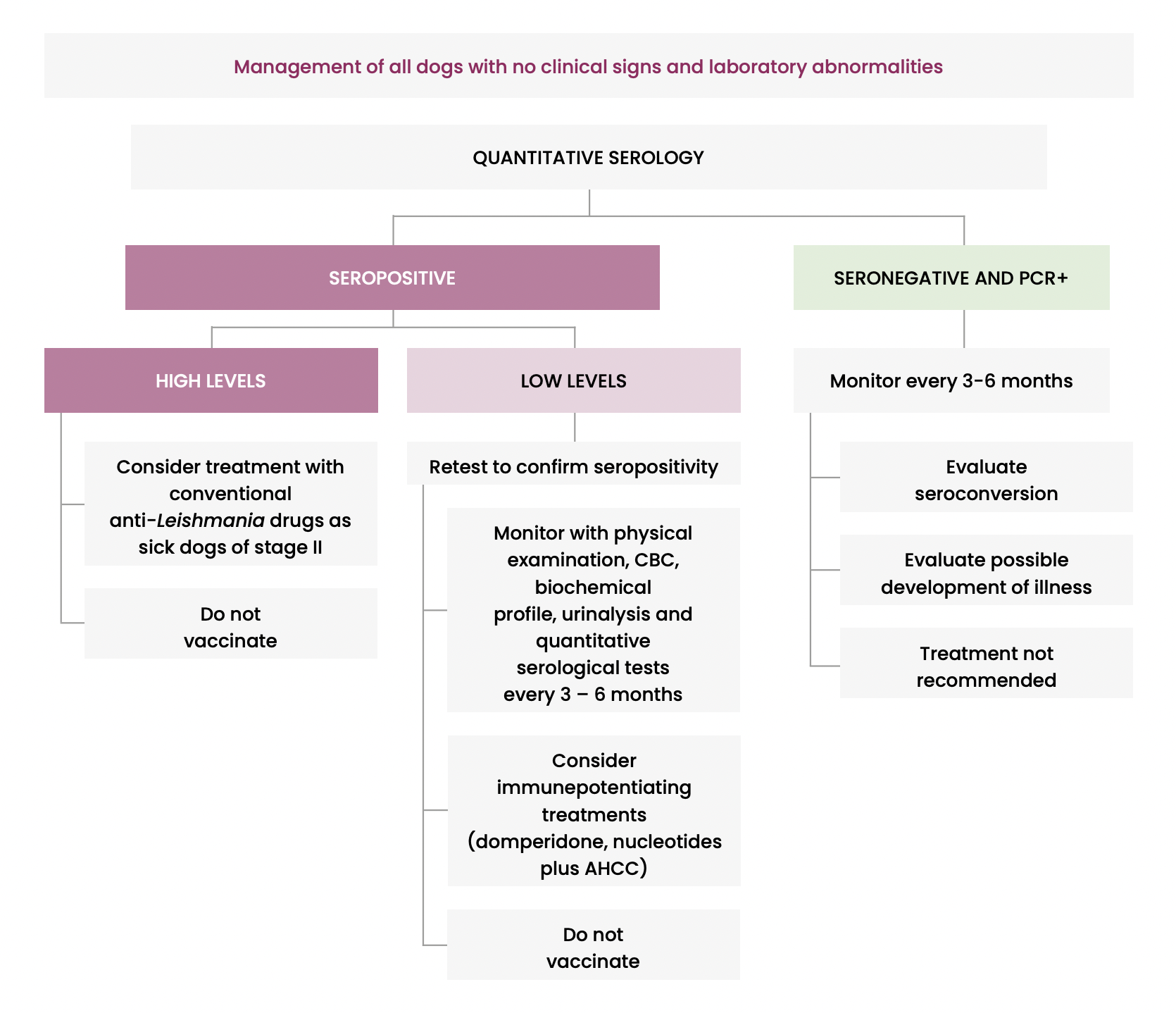
It is recommended to use serology alone or the combination of serology with PCR for screening healthy dogs and to avoid screening clinically healthy dogs (not vaccinated) only by PCR.